SaaS provider Veridat is working on helping the pharmaceutical industry to maintain the highest level of quality through their SaaS platform, Bench.
Bench makes it effortless to capture critical time-dependent information to the BSV blockchain, to provide what they call ‘Trust as a Service’. From pre-clinical trials through production and even distribution, Veridat verifies the occurrences of events and the veracity of data every step of the way.
How verifying pharmaceutical data via a public blockchain improves confidence in drug manufacture
All parties share an interest in the integrity of the data manufacturers deal with and emit. Data is commonly sourced via contract research organisations and collated from different silos.
In order to assure the development and use of a safe and efficacious drug, a host of different parties must come together with trust – from drug startups working on lead development, contract research organisations doing preclinical testing, to pharma drug development, to institutions performing clinical trials, the FDA approving it, to the doctors prescribing it and the customers taking it.
How can these companies possibly ensure the data is unadulterated so they can make smart decisions to either advance drug development or to pump the brakes on it as a regulatory body? Do we have all of the information from the manufacturer and is it accurate as a whole?
That is a big arc to cover and shining a light on all aspects in a verifiable and immutable manner should help generate a level of trust that currently does not exist.
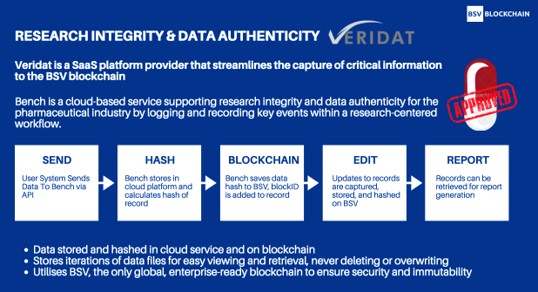
Veridat’s service solves data integrity concerns for both manufacturers and consumers. Their API, when integrated, works effortlessly in the background capturing information as it occurs in real time. It hashes the information to the BSV blockchain so the timestamped data verifies that a particular event occurred at a specific time and was created from a particular identity.
Here’s how Veridat’s data verification works: if somebody were to create a document, they can check that document against Veridat’s own hash generator that they’re getting the information they’re supposed to get. Regulators can be 100% sure that the information they’ve received is accurate, as the manufacturer’s data should match with Veridat’s.

According to Katherine Eban, author of ‘Bottle of Lies: The Inside Story of the Generic Drug Boom’, the overseas production of generics and active ingredients has proven susceptible to fraud. It is difficult for the FDA to use its usual tools, such as surprise audits, in a foreign jurisdiction.
Within the US examples of fraud in the drug industry are quite rare, but the public trust in FDA processes and pharma operations is in dire need of strengthening (as we have seen in this pandemic).
Blockchain technology can help create more trust in the drugs that are released in a proven marketplace to help curb instances of fabrication and fraud.
How Veridat’s solution can help government agencies make better decisions about drug approval
Veridat recently presented their solution to the USA’s FDA, the Food and Drug Administration. They discovered that the FDA had already explored solutions based on blockchain technology, but they were looking at a private blockchain, which is not much more than a file server. FDA officials recognised that Veridat’s reporting system interfaces with data stored on a public blockchain, letting them verify data that occurred during the manufacturing process or during clinical trials straight on the blockchain. Veridat’s interface makes it simple for pharmaceutical manufacturers’ to grant regulators access to their records, giving them a complete, comprehensive and verifiable view of the exact information they need.
All Veridat’s records are recorded to the BSV blockchain, making it easy to search, sort and filter through to find exactly what is needed within minutes instead of sorting through massive piles of paperwork. FDA officials were very receptive to it, and invited Veridat to several other working groups, both within and outside of the FDA.
What’s next for Veridat
Veridat is more than an idea. The company has run a year-long prototype with a top tier pharmaceutical company during which the Veridat system has performed well. Based on this early success, the company is now expanding past testing and recruiting additional industry partners.
